- OBG Access Newsletter
- Posts
- Introducing the OBG Access Commercial Platform!
Introducing the OBG Access Commercial Platform!
A smarter, faster way for obstetric medical-device companies to commercialize in the US market

Read time 6 minutes
In this month’s newsletter, I share details on a project I’ve been working on for the past 12 weeks.
An online platform that offers a smarter, faster way for women’s health and obstetric medical-device companies to commercialize their product in the US market.
I know first hand the commercial barriers that early stage companies often face: fragmented hospital data; slow pilots/trials; long sales cycles; limited decision maker connections; and an unclear roadmap from regulatory clearance to commercial adoption.
The OBG Access Commercial Platform has been designed to address several of these key challenges.
Most of my readers will already know - I was a co-founder of Safe Obstetric Systems where we developed and commercialized an obstetric medical device (Fetal Pillow) over a 10 year period, before being acquired by an industry leading player (CooperSurgical) in early 2021.
On reflection, I believe there are several tools we could have benefitted from, had these been available. I recently partnered with a software developer in New York to take on the task of building these tools myself.
We conducted multiple interviews with potential users (including startup founders, CEOs, distributors, industry experts, and sales professionals) to identify key features to be incorporated into an initial version of the platform.
My goal was to create a central hub that offers access to our experience, insights, and commercial network - built over many years.
I’m excited to announce that the platform is now live. I’m sharing details here for the first time, to subscribers of my OBG Access Newsletter.
Below is a first look at the five core features of the platform, designed to accelerate commercialisation and adoption in the US market.
At the end of the newsletter, I share detail on how you can access these tools.
1. Curated US Hospital Directory
The OBG Access platform includes a regularly updated, obstetrics-focused national hospital directory, built specifically for innovators bringing new L&D, postpartum, and OBGYN technologies to market.
Currently this directory includes the top 10 states (by birth volumes) and the top 10 individual facilities within each.
In aggregate, you have visibility of more than 100 curated hospital profiles, and the following additional information on each:
Annual birth volumes (including data sources)
Department Leadership contacts (OB Chairs, Labor & Delivery Directors, and Directors of MFM)
Direct links to clinical leadership pages (if available)
“Warm Introduction” - a flag to highlight where I can make a personal introduction to the clinician or facility (this will be dependent on the stage of your business/ product)
“Adoption Rating” - a proprietary score that grades how likely a hospital is to adopt a new medical device within their OBGYN department
Academic vs. community vs. system-affiliated categorisations
Parent Systems Directory - the largest systems/ IDNs and the level at which each makes purchasing decisions (centralised, regional, or hospital level)
Hospital Map - this plots hospitals from the directory in a map view, allowing you to visualize your territories and plan your logistics
The above data offers a valuable starting point to build a go-to-market plan, identify key US facilities to target, and determine which clinicians to engage with.
From personal experience - working directly with decision makers from the start makes a huge impact on driving adoption.
The result is faster targeting, smarter segmentation, and fewer months wasted starting conversations with the wrong hospitals and clinicians.
I have included a few screenshots below of each directory. This dataset will be regularly updated as I continue to engage with US hospital groups and clinicians.

US Hospital Directory: High Volume Facilities

US Parent Systems Directory
2. US Pipeline Tracker
After identifying target facilities from the Hospital Directory, you'll need a central hub to manage your engagement with each account, and most importantly to track your progress through the sales cycle.
During our US launch, we utilized a handful of CRM tools, but felt they were overcomplicated for our needs (designed for all industries, rather than the niche field of medical devices within obstetrics).
We would often revert back to multiple spreadsheets to track our sales activities across the team (which was also far from ideal).
Commercialisation can slow when you lose visibility across dozens of simultaneous pilot sites, clinician conversations, evaluations, and sales stages.
I believe a simple, centralised hub, accessible to all of your team members offers a valuable resource to manage all sales activities across the US market.
The Pipeline Tracker tool is your built-in, lightweight CRM specifically designed for sales cycles within the OBGYN field.
It offers a dashboard for visibility of all accounts at once, their stage within the sales cycle, and their estimated annual contract value. An overview as follows:
A simple CRM-style process built for medical devices in the OBGYN field
5 distinct sales cycle stages based on our experience bringing an obstetric innovation to the US market
Several fields to collect data on engagement: clinical champion; sales cycle stage; key contact information; important dates; next actions; and account notes that can be shared with other team members
Table or Kanban view, depending on your preference
Real-time dashboard with a view of overall progress in the market
Whether you’re managing 5 pilot sites or 50, this platform provides a central view of commercial progress and highlights where you should be focusing your efforts to drive adoption in the market.

Dashboard for overall view of commercial progress

Table view of all accounts in the pipeline

Kanban view of accounts in the pipeline
3. Distribution & Sales Partners Directory
This database helps identify potential distribution and sales partners in the US market.
It includes listings for specialty distributors and independent sales reps with established OBGYN relationships.
These have been vetted by me personally. Growing your US footprint requires the right partners, but many companies don’t have the network to find them.
This tool is currently under development and will include the following details on potential commercial partners to support your go-to-market strategy:
Independent distributors
Fractional sales reps
Clinical educators
Consulting groups
Former OBGYN hospital reps
Each profile will include:
Contact details
Territory coverage
Experience within L&D, postpartum, fertility, or minimally invasive GYN devices
Coverage areas, specialties, previous device portfolios, and availability
Relevant relationships with major IDNs
Links to profiles (LinkedIn + professional bio)
This directory saves months of cold outreach and accelerates the formation of a scalable, flexible commercial team.
It’s a tool we would have benefitted from when preparing for our US launch.
If you’re reading this newsletter, fit the criteria above, and would like to be added to this directory as an opportunity to connect directly with startups - please reach out for a conversation.
4. Conferences and KOL mapping
Medical device companies often struggle to decide where to exhibit, who to meet, and how to create meaningful impact at clinical conferences.
I shared our experiences and insights from attending conferences over the years in a prior newsletter: Conferences & Commercial Success.
The OBG Access Platform Conference Tool offers the following:
A curated list of key events (ACOG, SMFM, AWHONN, Regional Perinatal Quality Collaboratives etc) where you might consider demonstrating your innovation
Conference tags, topics, and focus areas (to identify where is most relevant for your technology)
Summaries of high-value sessions within each event that might be relevant to emerging technologies in the field
Featured Speaker lists to identify clinicians within your area of focus
KOL spotlight lists for each meeting, including OB Chairs, MFMs, and L&D Directors
The Conference Tool will allow you to develop a strategic plan, before you step into the convention centre to ensure your resources are being used effectively.
This was especially important for us as a UK company entering the US market - we had to be laser focused with our time and resources when deciding which US conferences to attend.
Below is a snapshot of the conference listing page - clicking into the “View Details” will open up a specific page relevant to that meeting, including a host of information as described above.

US OBGYN Conference Directory

District II Annual Meeting Details (past conference)
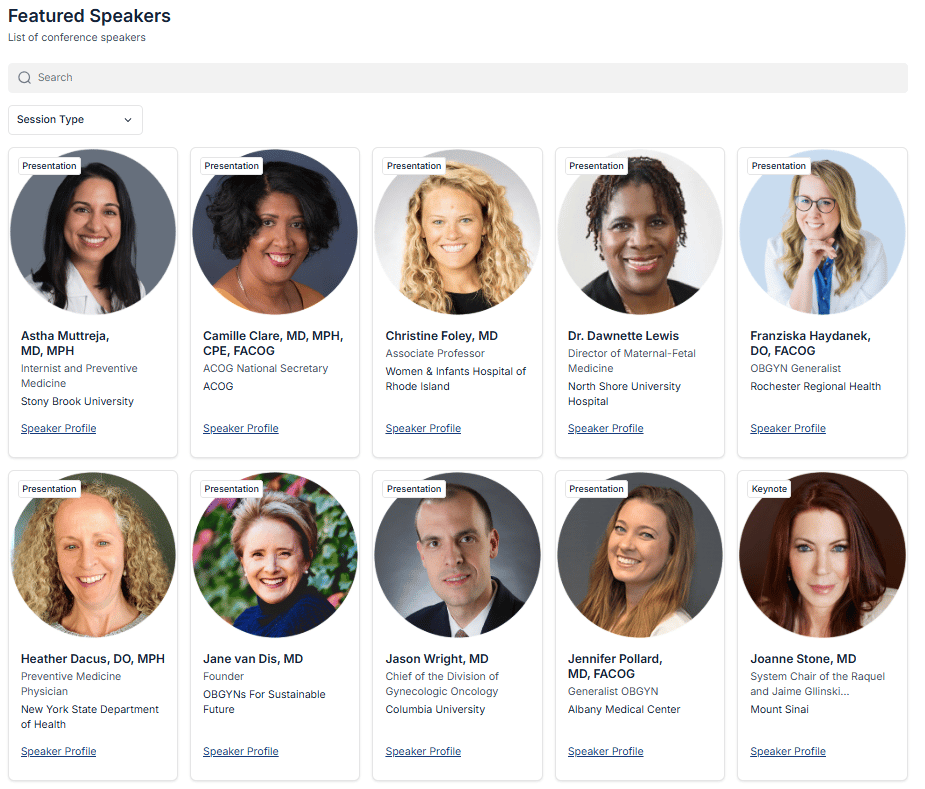
District II Annual Meeting Featured Speakers (past conference)
5. Commercial Resources & Tools
The Resources Hub includes strategic content, built from real-world experience.
To support teams navigating their US commercial launch, or scaling an existing one, the platform includes a growing library of practical resources such as:
Unique Insights from our journey with Fetal Pillow (from concept through to exit)
GTM Checklists (from launching your first US pilot to gaining VAC approval)
Podcasts & interviews (hear from others who’ve successfully navigated this journey before)
Commercial Roadmaps (e.g. navigating the US sales cycle, sales presentations, driving adoption etc)
Hospital procurement pathway maps
Clinician value-proposition templates
Exit readiness checklists for companies preparing for a future acquisition
Strategic Partner Profiles and M&A Activity within the market

Resources & Tools Hub
Interested in a demo of the OBG Access Platform?
This platform has been built from real-world experience launching an obstetric innovation into the US market, before being acquired by an industry leader.
It brings together the datasets, contacts, and tools that founders typically take years to build - and makes them available from day one.
If you’d like a demo, please reply to this email to register your interest.
I’ll be scheduling meetings during January where I’ll take you through through the platform and answer any specific questions you might have.
Together, let’s accelerate innovation in women’s health!
All the best,
Nish